Microelectronic circuit components are formed by diffusion of impurities into the epitaxial layer.
Areas over which a particular diffusion is required are defined by an oxide layer with windows cut in it.
Areas with oxide inhibit diffusion while areas without allow impurities to diffuse through it.
The oxide used for masking diffusion is usually silicon dioxide.
Besides, the use of silicon dioxide as a diffusion barrier for selecting doping silicon. Other uses of silicon dioxide include:
1. Dielectric (insulator) for MOS devices.
2. Passivation and Protection of the silicon wafers.
For oxidation, cleanliness is targeted to the molecular level.
The RCA clean is implemented before oxidation to remove the following:
1. Organic Contaminants
2. Trace Metals
3. Alkali Ions
Silicon wafers are oxidized by placing them in a high temperature furnace (900°C < T < 1200°C) in the presence of oxygen or water.
By controlling temperature and oxidation time precisely, oxide thickness can be predicted and controlled accurately.
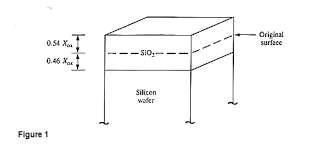
In oxide layer growth process, the oxide expands to fill 56% of the region above and 44% below the original surface.
There are two types of oxidation which are:
1. Dry Oxidation (1000°C - 1200°C) Equation : Si + O2 => SiO2 + siH2
2. Wet Oxidation (900°C - 1000°C) Equation: Si + 2H2O => SiO2 + 2H2
Mathematical modeling has shown that the relationship for long oxide time is parabolic while for short oxide time, the oxide thickness versus temperature is linear.
For long oxidation times, the process may be modeled by a simple equation known as the parabolic growth law which is x02 = Bt
where:
x0 = Thickness of the growing oxide
B = Parabolic Rate Constant
t = Oxidation Time
Short oxidation times can be modeled by another simple equation known as the linear growth law which is x0 = C(t + λ)
where:
C = Linear rate constant
t = Oxidation Time
λ = Initial time displacement to account for the formation of the initial oxide layer at the start of the oxidation process.
Factors that influence oxide growth:
1. Temperature: The growth rate of the oxide increase with the temperature
2. Moisture: Silicon dioxide grows better in steam because water has a much higher solubility than dry oxygen in silicon dioxide. Thus, water vapour or steam makes thicker oxide. When a thin oxide is needed dry oxygen is used.
3. Duration: The thickness of the oxide increases with time.
4. Crystal Orientation
5. Pressure
No comments:
Post a Comment